A major contribution of the Notify project was the participation of a diverse group of professionals who come from different disciplines and who ordinarily do not communicate with one another. Transplant surgeons, orthopaedists, ophthalmologists, transfusion doctors and haematologists, infectious disease specialists, pathologists, nurses, embryologists and gynaecologists, eye bankers, blood and tissue bankers and regulators had the opportunity to interact and provide their own perspectives. From these discussions some common definitions that can be applied across all fields were agreed upon whilst others are under discussion.
The following definitions adopted in the European Directives for Tissues and Cells and for Organs and blood were considered appropriate and useful for international application, although they were mapped to less technical language to improve accessibility by the general public:
1. Directive 2004/23 defines a Severe Adverse Event (SAE)* in the context of tissues or cells as any untoward occurrence, associated with the chain, from donation to
transplantation that might lead to the transmission of a communicable disease, to death or life-threatening, disabling or incapacitating conditions for patients or which results in, or prolongs, hospitalization or morbidity. Similarly, Directive 2002/98/CE defines Severe Adverse Event (SAE) for blood components as any untoward occurrence associated with the collection, testing, processing, storage and distribution, of blood and blood components that might lead to death or life-threatening, disabling or incapacitating
conditions for patients or which results in, or prolongs, hospitalisation or morbidity. In the Notify project these are referred to as cases of ‘Risk of Harm’.
2. For tissues and cells in the EU a Severe Adverse Reaction (SAR) is any unintended response, including a communicable disease, in the living donor or in the recipient that
might be associated with any stage of the chain from donation to clinical application that is fatal, life-threatening, disabling, incapacitating, or which results in, or prolongs,
hospitalization or morbidity. Similarly, Directive 2002/98/CE defines Severe Adverse Reactions (SAR) for blood components an unintended response in donor or in patient
associated with the collection or transfusion of blood or blood components that is fatal, life-threatening, disabling, incapacitating, or which results in, or prolongs, hospitalisation
or morbidity. In the Notify project, these are referred to as cases of ‘Harm to Donor’, ‘Harm to Recipient’ or ‘Harm to Fetus/Offspring’.
* Note however that this use of the term Serious Adverse Event is different from that in the context of pharmacovigilance, where (serious) adverse event refers to harm to the patient, which might or might not have been caused by the drug concerned.
3. Imputability of harm should be assessed, i.e. the extent to which the harm detected is likely to have been caused by the MPHO donation or clinical application. The assessment
should be based on available information and graded as: proven, probable, possible, and unlikely or excluded. The following Table 3 describes the possible outcomes of an
imputability investigation. A further category of ‘intervened upon without documentation’ has been used for organ transplantation situations where recipient treatment has
been applied prophylactically in the context of a known risk.
The stringent definition of proven or definite transmission should be used if the evidence is conclusive beyond reasonable doubt for attributing the adverse occurrence to the quality/safety of tissues/cells/ blood components (for recipients) or to the donation process (for donors). For example if there is clear evidence of the same disease in the multi-organ donor and at least one of the recipients. Absence of pre-transplant disease in the recipients should be documented. Variable involvement of different organs or tissues, different processing of organs and tissues, and recipient differences (i.e. pre-existing seroprotection or use of lymphocyte depleting induction in some but not all recipients) may
contribute to variable disease transmission.
The stringent definition of excluded can be applied if there is clear evidence of an alternative cause for the adverse occurrence. This may occur if there was pre-existing infection in multiple recipients but infection could not be identified in the donor or if testing of the same infection failed to document a clonal or donor-phenotype in the identified infection.
The term probable should be applied if there is evidence strongly suggesting but not proving that that an adverse occurrence was caused by the donation process or by the MPHO applied. Examples include if the same infection is documented in multiple recipients but not in the donor; or if there is epidemiologic evidence suggesting transmission (i.e. TB isolated from a recipient that types to a region where the donor lived, even if the donor tests are negative).
Imputability should be graded as possible for all situations where a) data suggest a possible transmission but are insufficient to fulfil criteria for confirmed transmission (proven and/or probable) or b) a transmission cannot be formally excluded. In the case of infectious transmissions, if only one recipient is available or other recipient(s) of the same donor cannot be appropriately tested, the maximum degree of indeterminate but probable transmission can be reached.If all or some of the recipients received an intervention (i.e. antimicrobial therapy or organ removal) and no disease was recognized in any of the recipients, the term intervened upon without documented transmission (IWDT) was utilised. If some but not all recipients had an intervention but disease transmission was recognized in even one recipient, this category should not be used.
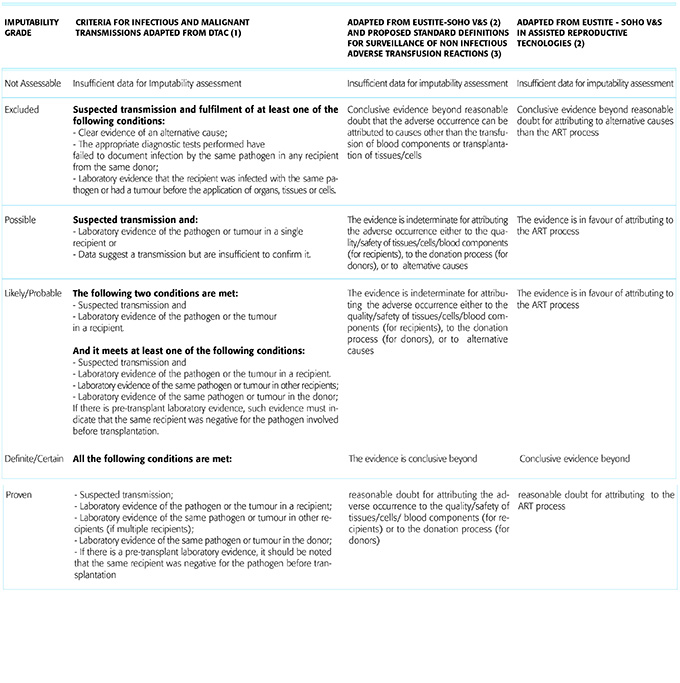
Table 3. Scale describing the possible outcomes of an imputability investigation in the case of harm to a recipient, a donor or a fetus/offspring.
(1) Uniform Definitions for Donor-Derived Infectious Disease Transmissions in Solid Organ Transplantation. Christian Garzoni and Michael G. Ison Transplantation • Volume 92, Number 12, December 27, 2011
(2) SOHO V&S Guidance for Competent Authorities: Communication and Investigation of Serious Adverse Events and Reactions associated with Human Tissues and Cells http://www.notifylibrary.org/sites/default/files/SOHO%20V%26S%20Communic...
(3) Proposed standard definitions for surveillance of non-infectious adverse transfusion reactions, incorporating correction to TRALI definition (as adopted June 2013). ISBT Working Party on Haemovigilance
